Abstract
Background:
The standard of induction therapy for fit patients(pts) with acute myeloid leukemia (AML) has not changed much since 1973, when the 7+3 regimen of cytarabine and anthracycline was born. This combination of agents produces complete remission (CR) in about 60-80% of younger adults and in 40-60% of older adults (60 years) depending on genetic risk group. However, compared with AML in favorable- and intermediate-risk categories, induction therapy for fit AML pts in adverse-risk category had significantly lower CR rates and dismal outcome. Recently, combination therapy with venetoclax and azacitidine or decitabine for the treatment of older pts with de novo AML unsuitable for intensive chemotherapy, has resulted in response rates of 60-70%. Currently, the role of venetoclax in combination with azacitidine or decitabine in young adults with newly diagnosed ELN adverse-risk AML remains unclear. This study aims to evaluate the safety and efficacy of venetoclax plus decitabine as induction therapy in young adults with newly diagnosed ELN adverse-risk AML.
Methods:
This is an interim analysis of an ongoing phase 2 clinical trial (NCT04752527) of a planned 42 untreated ELN adverse-risk AML pts 18-59. The fast next-generation sequencing (NGS) for the most commonly mutated genes in AML using Oncomine TM myeloid research assay on Ion S5 and Chef platform (Thermo Fisher) was completed within 72 hours, which could screen for the ELN adverse-risk features in AML pts together with multiplex RT-PCR and fluorescence in situ hybridization (FISH). In cycle 1, pts receive decitabine 20mg/m 2 on d1-5 and venetoclax escalated from 100mg to 200mg to 400mg until 28-day cycle is finished. For pts with high FLT3-ITD allelic ratio, sorafenib was optional administered at a dose of 400mg orally twice daily. Bone marrow assessments were performed on d28 before subsequent cycle. Pts who achieve composite complete remission (defined as complete remission [CR], CR with incomplete hematologic recovery [CRi], CR with partial hematological recovery [CRh], and morphologic leukemia free state [MLFS]) receive 1 or 2 cycles of consolidation with high dose of cytarabine followed by allogeneic hematopoietic stem cell transplantation. Non-responders or pts who achieve partial remission (PR) receive 1 additional cycle of combination of venetoclax plus decitabine. The primary objective is to determine the composite complete remission rate (CR+CRi+CRh+MLFS). The endpoint is to show that venetoclax plus decitabine is superior to historical control (HC) of cytarabine combined with idarubicin (12 mg/m 2) in young adults with newly diagnosed ELN adverse-risk AML.
Results:
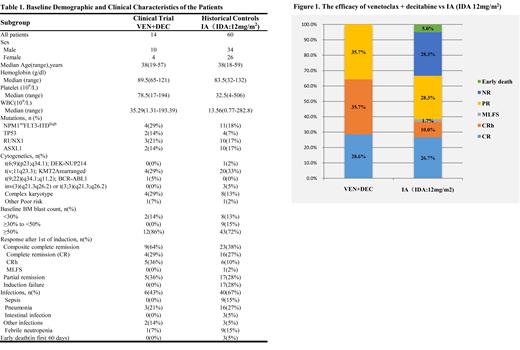
From February 1, 2021 to July 31, 2021, a total of 104 newly-diagnosed AML pts underwent screening, and 30 pts were classified as ELN adverse-risk category by NGS (within 72 hours) together with multiplex RT-PCR and FISH. Totally, 19 pts with ELN adverse-risk were enrolled, comprising 14 males and 5 females. 2 (10%) pts had secondary AML (sAML). Median age was 38 years (range, 19-57 years). The baseline patient characteristics are summarized in Table 1. Outcome data were updated as of July 2021, for a median follow-up of 2.7 months. Among 14 evaluable pts, 4 (28.6%) achieved a CR, 5 (35.7%) achieved a CRh and 5 (35.7%) had a PR in cycle 1. Individual pts data of this study (n = 14) was compared to a HC, combining individual pts data of AML with adverse-risk from the SZ3202 registry (n = 42) and the pts treated with IA (IDA:12 mg/m 2) in the same period (n = 18). The venetoclax plus decitabine cohort demonstrated higher rates of CR than the HC cohort, with an observed CR/CRh rate of 64.3%, compared with 38.3% (Figure 1).
The regimen was well tolerated, with 4- and 8-week mortality rates of 0% and 0%, respectively. The most frequent nonhematologic adverse events of any grade were neutropenic fever (n=6) and pneumonia (n=3). With a median follow-up of 2.7 months, the median remission duration and overall survival (OS) have not been reached. Tumor lysis syndrome occurred in one patient and resolved with medical management.
Conclusions:
Preliminary results indicate that venetoclax plus decitabine is an effective, lower-intensity regimen that is well tolerated for young adults with newly diagnosed ELN adverse-risk AML, producing high rates of CR, low rates of infections, and low rates of early death. Recruitment of young adults with newly diagnosed ELN adverse-risk AML pts for this trial is ongoing.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal